Electrolytes
introduction
Electrolytes are a term that you may not even know exactly what is behind it. They are found on some laboratory papers, sound terribly chemical and in fact their function and regulation are extremely complex.A simplified explanation of the medical context is given below.

definition
So-called electrolytes are salts dissolved in the blood. Table salt can be used as a comparison. If you dissolve table salt, which is chemically called sodium chloride, in water, the components of the salt, namely the sodium and chloride ions, separate from each other when they dissolve and are enveloped by water molecules and thus dissolved.
Certain salts are also dissolved in the blood as ions, the most important of which are sodium, potassium, Calcium and chloride. There is also, for example magnesium or Bicarbonate, however, these have different functions in the body and are less often included in a blood test. As the name electrolyte suggests, these ions are electrical charge carriers. Sodium, potassium, calcium and magnesium are positively charged, while chloride and bicarbonate are carriers of negative charge. These electrolytes ensure the chemical and electrical balance and are distributed throughout the body via the blood, where they are needed by every single cell to live and function.
function
Electrolytes have a complex function in the household of every cell in the body. They are particularly relevant for heart and muscle cells, in the kidney, in nerve cells and sensory cells, for example in the ears or eyes. The decisive factor is the electrical charge of the ions. In order to understand the complex mechanisms of a cell, one must bear in mind the following principles:
-
The predominant group of ions within the body's cells is potassium. Very little of it is found in the blood. Sodium, on the other hand, is mainly present in the blood and the space outside the cells and hardly within the body cells. Everything outside the cells (including the blood) is summarized as an extracellular space, because ions can spread and move in them without any problems.
-
Body cells and extracellular space are different compartments. An exchange of ions between them cannot take place without openings in the form of channels in the cell walls. There are sodium and potassium channels that are located in the cell membrane and are closed in their initial state.
-
Ions strive to want to spread evenly in their compartment. If a channel is now opened between the cell and the extracellular space, this driving force ensures that the ions flow to where there are fewer of them.
When a signal transmitter reaches a cell, the ion channels there are opened according to the lock and key principle and the ions can flow into the cells. This changes the electrical charge in the cell, because the ions bring positive charges with them. This change in the electrical charge sets other processes in motion in the cell, which differ from cell to cell depending on the function. The ions that have flown in are then transported outside via a pump in the cell membrane in order to restore the initial state.
Another function of the ions is to bind water. The higher the salt content, the more water it attracts, this principle is called osmosis. This plays an important role especially in the kidneys and also explains why a low-salt diet is recommended for patients with high blood pressure anyway.
Read more on the subject below Diet for high blood pressure
In summary, the individual electrolytes can be roughly assigned to certain organ systems for which a balance is essential. Potassium is important for the heart muscle, sodium for the kidneys and blood pressure, calcium for the bones and heart, magnesium for the muscles and the brain, and bicarbonate for the pH value, i.e. the acid-base balance of the blood.
Read more on the subject below Acidosis
Importance of blood for electrolytes
The blood is the main transport route for electrolytes. Every cell in the body is reached via the blood vessels and small capillaries. The blood collects the electrolytes that we have ingested through food or fluids in the intestines and distributes them in the body where they are needed. The kidney is the filter that uses various regulatory mechanisms to decide which electrolytes are still needed in the body and which can be excreted in the urine. The electrolytes in a blood sample can be used to determine the balance of the body. Many diseases can be read off very well from the electrolyte values.
Read more on the subject below
- Laboratory values
- Electrolytes in the blood
Some normal values are listed below, but they may differ slightly from laboratory to laboratory:
electrolyte | Lower limit in mmol / l | Upper limit in mmol / l |
sodium | 135 | 145 |
potassium | 3,6 | 5,2 |
Calcium (total) | 2,20 | 2,95 |
magnesium | 0,73 | 1,06 |
chloride | 98 | 106 |
Bicarbonate | 22 | 26 |
The most important in diagnostics are sodium, potassium and calcium. They are mostly controlled by hormones. They are the most sensitive, the quickest to lose their balance, and the most serious consequences. Sodium and potassium are obtained via the hormone aldosterone (a so-called. Mineral corticosteroid) which is released from the adrenal cortex, whereas calcium is controlled by the parathyroid hormone from the parathyroid gland. Both hormones send their signals to the kidneys as to whether the electrolytes should be excreted when they are in excess or retained in the body when they are deficient. However, if there are disturbances in this control loop, e.g. Certain medications, diseases of the hormonal glands or deterioration in kidney function shift the electrolyte balance, which is noticeable in the body.
Read more on the subject below
- Adrenal hormones
- Parathyroid hormones
Another cause of electrolyte shifts is an increased accumulation of electrolytes. Potassium, which is released from dying cells, is mainly used for this. This is not a problem with individual cells, but if a larger amount of tissue is lost, this can definitely have an impact on the electrolyte balance. This is the case, for example, with tumor diseases (so-called tumor lysis syndrome) or with frostbite or burns of larger parts of the body, so that a large excess of potassium occurs.
Read more on the subject below Hyperkalemia (excess potassium)
Deficiency and Consequences
Not only the deficiency, but also a shift in the equilibrium of the electrolytes or an excess of a certain electrolyte can have serious consequences depending on the extent.
A lack of sodium manifests itself in drowsiness, confusion and also nausea. If, on the other hand, there is an excess of sodium in the blood, seizures comparable to epileptic seizures up to coma can occur.
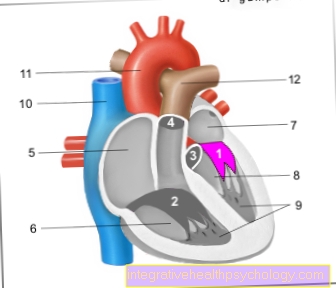
Changes in the potassium level are particularly noticeable in the heart. If you have less than 3.6 mmol / l potassium e.g. Certain medications, such as diuretics ("water tablets"), can cause constipation, muscle weakness with decreased reflexes, abnormal sensations and numbness of the skin. If you have more than 5.2 mmol / l, the reflexes tend to be increased, but it can also lead to temporary paralysis. However, the most important consequence of a potassium deficiency or excess is cardiac arrhythmia. Potassium is essential for the transmission of signals to the heart. If this balance is disturbed, ventricular fibrillation can even occur!
Read more on the subject below Recognize potassium deficiency
Calcium is also important for the heart, but cardiac arrhythmias do not occur quite as often with an excess of calcium as with potassium. If you have too much calcium, this is mainly noticeable through nausea and vomiting, kidney stones, bone pain and muscle weakness. Too little calcium manifests itself in a tingling sensation on the skin, especially in the face, and muscle cramps in the hands and feet (so-called tetany with paws).
If you have too little magnesium, this is symptomatically similar to the calcium deficiency e.g. with muscle spasms, but neurological symptoms such as you experience delirium or temporary heart failure. Too much magnesium is often not expressed at all, possibly leading to drowsiness.
Read more on the topic: You can recognize a magnesium deficiency by these symptoms
Chloride ions hardly play a role in diagnostics because they are bound to sodium by regulation. If there is an imbalance, the sodium is particularly affected, which is primarily symptomatic.
Bicarbonate mainly plays a role in the acid-base balance, whereby the bicarbonate takes over the function of the base. A deficiency occurs, for example, in diarrhea, when the body loses a lot of bicarbonate. The result is an over-acidification of the body, which can, however, be partially compensated for by counter-regulation. There are hardly any serious consequences.
Learn more about the Causes and consequences of an electrolyte deficiency.
Electrolyte balance

With the unauthorized filling of electrolytes one should careful be. The symptoms are often very unspecific and cannot necessarily be attributed to an electrolyte disorder without checking the blood values. Should e.g. If a severe electrolyte disturbance is noticed during a hospital stay, this can be through Infusions or Medication be balanced.
However, topping up electrolytes yourself is particularly advisable in one situation, namely when Diarrheal diseases. One often loses a lot of electrolytes through frequent visits to the toilet or through vomiting. In order to refill these, there are ready-made electrolyte solutions in powder form to buy in the pharmacy. They are ideal for restoring the electrolyte balance, and you often feel much better after taking them.
So-called isotonic drinks can also be useful in competitive sports with high water loss during sweating.
Electrolyte shifts can also be prevented, with the corresponding consequences, by avoiding foods that contain a lot of potassium, for example if you have kidney disease Bananas or Dried fruits.