Pentalong®
Active ingredient: pentaerythrityl tetranitrate
Mode of action

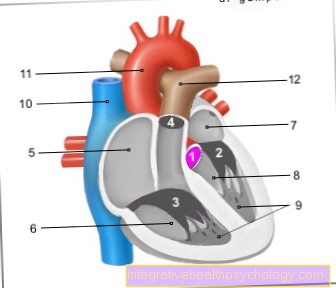
The active ingredient belongs to the group of vasodilating substances (Nitrates) and is used to promote blood flow to the heart in cases of constricted coronary arteries.
In the organism, the active ingredient becomes the body's own nitrogen monoxide (NO) reduced. This has a direct widening effect on the vessels.
Due to the improved blood circulation, the heart is better supplied with oxygen and attacks of chest pain (angina pectoris) are prevented.
In contrast to other drugs from the group of nitrates, Pentalong® should neither lead to nitrate-related headaches nor to the development of tolerance.
Pentalong® was previously sold in doses of 80mg and 50mg. However, since May 20, 2014, the drug has only been approved in the 50 mg dosage, as extensive studies have not shown sufficient effects of the higher dosage in patients with angina pectoris.
The drug is only available on prescription. It is suitable for prevention and long-term treatment.
Ingestion
Patients take unless otherwise prescribed 2-3 tablets daily Pentalong® 50mg whole about half an hour before eating.
The treating doctor determines the duration of the treatment or a different intake.
If an overdose is suspected, the treatment should be discontinued and a doctor should be consulted immediately.
If a tablet has been forgotten, do not take a double dose, but continue with the medication at the next regular intake time.
Side effects
Patients complain as a very common side effect (more than 1 in 10 patients treated) a headache.
Frequently (1 to 10 patients in 100) it can also cause unwanted symptoms, such as low blood pressure, Circulatory regulation disorders With collapse, dizziness, Drowsiness and fast heartbeat come.
Occasionally (affects 1 to 10 users in 1,000), it happens fleeting reddening of the skin (Flush) and allergic skin reactions.
Occur less often (1 to 10 users in 10,000) nausea and Vomit, skin rash, Cardiac arrhythmias or shortness of breath on.
Very rare (less than 1 in 10,000 patients) have been observed as a result of taking Pentalong® severe inflammatory skin diseases (e.g. exfoliative dermatitis, Stevens-Johnson syndrome, angioedema).
As soon as these or other symptoms are noticed, a doctor should be informed or consulted immediately and the drug should not be taken again.
Interactions
When taking other drugs in addition to Pentalong®, it must be noted that the Effect of the drug thereby possibly reinforced can be. This applies, for example, to taking at the same time Beta blockers, Calcium channel blockers, Diuretics, ACE inhibitors, Phosphodiesterase inhibitors, certain Antidepressants and Neuroleptics, as well as for Alcohol consumption.
Pentalong® also leads to a Weakening the effect of Heparin preparations.
In contrast, preparations from the group of Ergot alkaloids (e.g. dihydroergotamine) increased when taking Pentalong® hypertensive.
Contraindications
Pentalong® must not be used in Allergy / intolerance to the active ingredient, acute cardiovascular failure, shock, Heart attack, very low blood pressure (systolic value <90mmHg), as well as parallel intake from Phosphodiesterase inhibitors for the treatment of erectile dysfunction (e.g. Sildenafil, Vardenafil, Tadalafil), as this can lead to a marked drop in blood pressure.
In other specific circumstances, the doctor must carefully check whether it is safe to take Pentalong®. So for example. at increased intracranial pressure and numerous Heart disease (MyocarditisConstriction of the heart valves, thickening of the heart muscle, pronounced weakness of the heart muscle, fluid accumulation in the pericardium).
Use during pregnancy and breastfeeding
In the pregnancy and Lactation should the drug not be taken, as there is not yet sufficient knowledge as to whether the application is safe during this time.
Use in children and adolescents
There is currently insufficient knowledge about the safety of Pentalong® in children and adolescents. Hence the medicine is used in young people only after carefully examining the necessity to use.
Warnings
After taking Pentalong® you can Reactivity limited be.
Therefore, when participating in road traffic or when Operate machines greatest Attention required. this applies especially at the beginning of treatment, at Dose increase and related to Alcohol consumption.
The drug is neither for treatment one acute angina attack, yet to treat a acute heart attack suitable.
It also contains Pentalong® Lactose (Lactose), to which people with corresponding intolerance may have an allergic reaction.
background
Developed became Pentalong® in the 1950s in the US.
Since 1964 it was produced in the former GDR by a company from Zwickau.
Today has Actavis the rights on Pentalong®.
Since the drug never had to go through an approval process, Actavis had to apply for a subsequent approval. This was the procedure for all medicinal products that came onto the market before 1976, since their quality, effectiveness and safety were to be checked.
However, Actavis did not meet the requirements set by the Federal Institute for Drugs and Medical Devices, so that the Admission of Pentalong® 2005 initially expired.
Actavis sued this decision and subsequently submitted studies that were supposed to prove the effectiveness of Pentalong® 80mg. The studies were not convincing, so the The approval of this drug finally expired in 2014.
For the lower dosage with 50mg still exists fictitious admissionso that this drug remains marketable.
It will, however no longer reimbursed by the statutory health insurance. The patient has to pay himself.
Therefore, in many patients Changeover the medication of Pentalong® to other medicines containing nitrates performed. Alternative active ingredients are for example Isosorbide dinitrate (ISDN) and Isosorbide mononitrate (ISMN), as well as if necessary Nitro sprayswhich also belong to the group of nitrates and thus have a vasodilating effect.