Zostavax® vaccination against shingles
Introduction - What is a Zostavax® vaccination?
The Zostavax® vaccination is a vaccination that was approved in 2006 and has been available in Germany since 2013, which prevents the development of a belt rose (Herpes zoster infection) should prevent. In Germany, the vaccination against varicella zoster has been recommended since 2004 (chickenpox) with children.
The Zostavax® vaccination is aimed at the group of people over 60 years of age. Basically, the same pathogen is vaccinated against as with chickenpox. Interestingly, the immunity against an infection with varicella zoster does not persist after a vaccination or after an infection, but instead decreases again over the years.
It was found that the titer (concentration) of protective Antibodies from the age of 50. Because of this, if the titer falls below a certain value (concentration), reinfection (renewed infection) with varicella zoster is possible and herpes zoster (belt rose) can occur. The vaccination with Zostavax is supposed to counteract this waste, the protective antibodies, and thus protect against a new infection in old age and prevent a belt rose.
You might also be interested in: Causes of Shingles

Indications for a Zostavax® vaccination
The indication for a vaccination with Zostavax® lies in the prevention of the appearance of a belt rose (herpes zoster) and the sequelae of (post-therapeutic neuralgia). This occurs in people aged 50 and over at an increased risk.
Overall, between 25% and 30% of all people will develop shingles at some point in their life. Since the protective titer, i.e. the concentration of antibodies, decreases from the age of 50 (as explained in the introduction), there is an indication for vaccination with Zostavax® for people aged 50 and over. The indication is therefore prevention. If there is an active belt rose, the vaccination must not be used.
Read also: How contagious is shingles?
Contraindications - When should a Zostavax® vaccination not be given?
An absolute contraindication to carrying out the Zostavax® vaccination is the existence of a congenital or acquired weakness of the immune system. This can include diseases from the area Bone marrow and des lymphatic system be. This also includes chronic infections, such as HIV or tuberculosis. Also, patients should take higher doses cortisone should not be vaccinated with Zostavax.
In the group of immunosuppressed patients, vaccination with a subunit vaccine may be possible. These are dead vaccines - this means that only certain parts of the pathogens (antigens) are present in the vaccine. The vaccine has been here since October 2017 Shingrix® approved Furthermore, the Zostavax vaccination is not approved during the pregnancy and breast feeding period. In patients with one allergy If certain components of vaccines are suspected or known, they should only be vaccinated after a more detailed investigation - possibly also with the manufacturer.
In addition, vaccination is not given during an active herpes zoster infection or shingles.
What effect can be expected?
The active ingredient in the Zostavax® vaccination are living varicella-zoster pathogen. These are no longer able to trigger an infection. These are weakened forms of the pathogen - so-called attenuated pathogens.
In people whose immune system is no longer adequately functional, however, this live vaccine could lead to a course of an infection - therefore these are only used in healthy patients. When vaccinated with Zostavax®, no direct effect can be felt for the patient.
In Germany it has Robert Koch Institute (RKI) No recommendation has yet been issued with this vaccine. This decision is not based on the dangers or risks of the vaccination, but because of its lack of effectiveness. In its evaluation, the RKI came to the conclusion that the vaccine does not provide long-term protection against a belt rose (herpes zoster) through the Zostavax® vaccine.
In view of a cost-usage list, no recommendation was given. Particularly in the case of patients over 80 years of age, there was a lack of relevant data that would have proven its effectiveness. In control studies, it has been proven that the occurrence of a belt rose was reduced when vaccinated with Zostavax® compared to people who were vaccinated with a placebo. This shows an effectiveness of the vaccination of 70% (50-59 years) and 41% (70-79 years).
The data on how long the vaccination has been effective is less positive. After only two years, the effectiveness of the protection against shingles of less than 50% is again evident. From this, the RKI calculates a high cost factor for permanent - i.e. repeated - immunization (vaccination) with the vaccine. Interestingly, in other countries there is a recommendation for vaccination one year after the appearance of herpes zoster. This is to prevent recurrence of shingles.
Side effect of the Zostavax® vaccination
People who have received a vaccination with Zostavax® often have redness, swelling, itching and pain at the injection site. Furthermore, overheating, headaches, bruises, fever, muscle pain or even skin rashes can often occur.
Occasionally, patients report nausea and swollen lymph nodes. Wheals at the vaccination site are rare. Very rarely (1 out of 10,000 people vaccinated) a chickenpox infection or shingles was caused by the vaccination.
The Robert Koch Institute rates the tolerance of the vaccine as good. The local side effects are only mild and do not last long.
Dosage of the vaccine
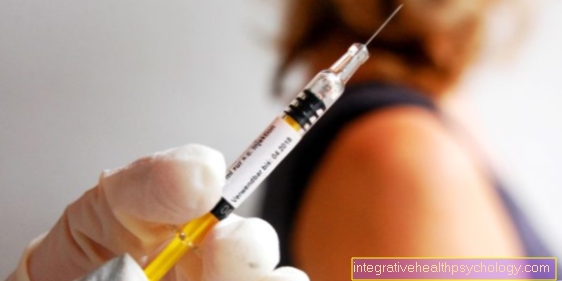
The dosage is specified by the manufacturer. The vaccine solution (0.65ml) is available on the market as a ready-made solution or powder. There are at least 19,400 pfu (plaque forming units) in it. This means the number of effective or active pathogens. The concentration in the Zostavax® vaccine is up to 14 times higher than that in the chickenpox vaccine, which is administered in childhood. The vaccination is carried out subcutaneously or intramuscularly in the lateral (outer) upper arm.
How much does the Zostavax® vaccination cost?
The cost of a ready-to-use dose of Zostavax® is available from the pharmacy for around 180 €. In addition to these pure "material costs", there is a vaccination fee to be paid by the treating doctor. According to the medical fee regulation, 4.66 € are charged for this. However, since this is a private treatment, higher costs may arise here. It can be assumed from about 7-8 €. The total costs for a vaccination are therefore around € 190.
Does the health insurance plan the vaccination?
All vaccinations required by the STIKO (standing vaccination committee) are recommended as relevant, are usually covered by the health insurance and the allowance. There is no recommendation for the Zostavax® vaccination. Accordingly, it cannot be assumed that the health insurance company will take over this. The rule here is that contacting the cash register can be helpful. In certain cases or with certain health insurers, there may be special regulations or individual special decisions that can lead to a complete or partial reimbursement of the costs. With various company health insurance funds, it is quite likely that the costs will be covered.
Alternatives to the Zostavax® vaccination
As an alternative to the Zostavax® vaccination, the Shingrix® vaccination approved on the German market. This differs in that it is not a live vaccine, but a so-called subunit vaccine. There are no living, weakened pathogens as an active ingredient in the vaccination, but parts of "surface structures". This vaccination can also be used for people with immunodeficiency. However, this should only be done after a precise and thorough examination of the approval and in consultation with the manufacturer. The effectiveness of the Shingrix vaccination shows in current studies of the Zostavax® vaccination clearly superior and very efficient.
Recommendations from our editorial team
- Shingles medication
- Course of shingles
- Shingles on the face
- Shingles pain
- The flu vaccination





.jpg)











-whrend-der-schwangerschaft.jpg)