pH in saliva
introduction
The pH value is a measure of how acidic or basic a liquid or substance is. A pH of 7 is called a neutral substance. Values below 7 are acidic liquids and values above 7 are basic liquids. Since saliva is made up of different components and is produced by different glands, its pH value can vary depending on its composition.

What is the normal pH in saliva?
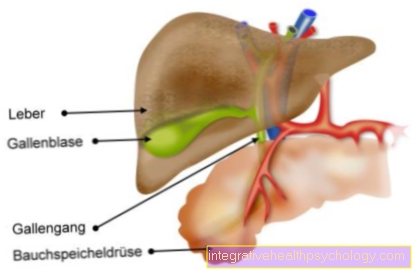
The saliva in our mouth consists of over 99% water and is produced by various salivary glands in the throat and mouth area. This includes, among others the parotid gland (Parotid gland), the sublingual gland (Sublingual gland) and the submaxillary gland (Submandibular gland). Each of these glands opens into the mouth with one or more ducts. They produce the saliva that is important to moisten our food so that we can swallow it better. For this purpose, saliva contains mucins, and there are also digestive enzymes such as alpha-amylase. It begins to digest carbohydrates in the mouth. Lysozyme is a bactericide from saliva that directly fights bacteria in the oral cavity. Another component of saliva is bicarbonate. It creates a slightly alkaline environment, that's how it is alpha-amylase particularly active and the tooth enamel is protected. Normally, saliva has a pH value that is relatively neutral (varies between 6.5 and 7.2 depending on the saliva secretion).
Does the pH value in saliva change during the day?
Since the pH of saliva is affected by food intake, it's no wonder it fluctuates over the course of a day. This is how it is in the morning before breakfast, because you haven't eaten for a long time overnight. After every meal, more saliva is suddenly produced and it becomes more alkaline. If the flow of saliva is shut down again after the meal and the new saliva replaces the old one, the pH value drops again.
Nowadays we generally consume acidic food (e.g. through fruit juices or similar), so it is an advantage that the alkaline saliva can balance the pH value in the mouth. In addition, the pH fluctuation depends on what the meal contains. A carbohydrate-rich diet (especially sugar) is more likely to lead to an acidic environment in the mouth than a protein-rich diet. So it causes greater fluctuations during the day.
What increases the pH?
The pH value in saliva reflects the acid-base balance throughout the body. If the pH value is too high, this indicates an alkaline metabolism. Then one speaks of a so-called alkalosis. This can be caused metabolically (i.e. by the metabolism) or respiratory (i.e. by breathing). Metabolic alkalosis occurs when you vomit often. Because then the body loses large amounts of stomach acid. This means that there are more bases in the body overall and the pH value increases. This chronic vomiting can e.g. occur in the course of bulimia. Other causes of metabolic alkalosis can include gastric irrigation, diuretic therapy (water tablets) or a Hypoalbuminemia (too little albumin in the blood) with liver failure or other diseases.
Respiratory alkalosis is caused by hyperventilation. The accelerated breathing causes the body to release more CO2. Since CO2 acts as an acid in the blood, its absence leads to an alkaline metabolism. Causes of reduced CO2 pressure in the blood can also be: altitude, pulmonary fibrosis, restrictive lung diseases and certain heart defects.
What are the long-term consequences of a pH that is too high?
A high activity of the salivary glands is accompanied by an increased pH value of the saliva. This can e.g. occur when one is constantly ingesting food. Then the salivary glands become more active and produce more saliva faster and more, so that the pH rises. Usually this is not a problem because the increased activity of the glands does not last long. A slightly higher pH even protects against tooth decay. However, if the pH value is constantly increased, this can also damage the teeth. In addition, the digestive enzymes in saliva can no longer work efficiently if the values deviate too much. Another reason for increased salivation can be the chronic vomiting associated with bulimia. The body tries to balance the acidic pH of the gastric juice in order to protect the teeth.
What lowers the pH?
If you eat food before measuring the pH (especially in the form of carbohydrates, e.g. sugar) and the mouth has not been cleaned (Bacteria can cause an acidic environment), the values can be falsified into acid. However, the pH value in the body can also be increased for other reasons, in which case one speaks of acidosis. This can also be metabolic (from the metabolism) or respiratory (from breathing) caused. Metabolic acidosis can occur in poorly controlled diabetics. Their blood contains more ketone bodies (acidic), which is called ketoacidosis.
Alcohol consumption and long periods of hunger (then there are more ketone bodies in the blood) are further causes. A lot of sport can lead to lactic acidosis in the short term (lactate = lactic acid). In chronic diarrhea, too much bicarbonate is lost and acidosis also occurs.
The acidosis in hypoventilation is respiratory-related, i.e. when too little CO2 is exhaled. This can e.g. in obstructive lung diseases such as bronchial asthma or bronchitis.
What are the long-term consequences of a pH that is too low?
In a diet rich in carbohydrates, the bacteria in the oral flora produce many acids. They can embed themselves in their own breakdown products and together with them they form a biofilm on the teeth, a Plaque. More and more acid is created, which now demineralizes the tooth. If the bacteria are deprived of their food for a short period of time, the tooth can often regenerate. If this does not happen, e.g. Due to constant carbohydrate consumption and poor oral hygiene, demineralization continues to progress. The consequences are holes in the teeth and tooth decay.
Is there an optimal pH?
The pH value in saliva should be slightly basic, i.e. around 7-8. From a pH of 6.7 the demineralization of the teeth begins and from 5.5 even the tooth enamel is attacked. When sugar is consumed, the pH is lowered by the acid that the bacteria produce. If you now have a fairly basic saliva as a starting value, the pH does not move so quickly into critically acidic ranges and can regenerate itself more quickly after eating.
The blood has an optimal pH value between 7.35 and 7.45. It is important that the pH of the blood is kept constant within narrow limits. Many enzymes can only work well enough in a very specific pH value to maintain the metabolism. In order to maintain the pH value between 7.35 and 7.45, there are various buffer systems in the blood that can compensate for too much acids or bases (phosphate buffer, bicarbonate buffer, hemoglobin buffer). If these buffers fail, the changed pH value is also reflected in the saliva.
How can you measure the pH value in saliva?
If you want to measure the pH value in saliva, for example to conclude that the body is over-acidic, you have to follow a few rules. The pH of the saliva depends to a large extent on how actively the salivary glands release secretions. Therefore, stimulating the glands (for example, eating food shortly before the measurement) would falsify the measurement result. The more active the glands are, the more basic the saliva becomes. This is due to the fact that with a faster flow of saliva there is less time to reabsorb sodium ions from the initially plasma-isotonic saliva in the ducts of the glands. It is therefore important not to eat or drink anything about two hours before the measurement. A sober pH value of the saliva can then be related to the pH value e.g. of the small intestine.
To measure the pH value yourself, you can buy pH test strips. Then you have to moisten the test strip with a little saliva, e.g. from the tongue. Then you rinse your mouth with lemon juice and then measure the pH value with a new test strip. For better test results, the measurement should be repeated a few times in two minutes (without a new lemon rinse).
How quickly the pH value rises (i.e. becomes more basic) is an indication of how good the blood's buffer function is. If the blood buffers well, it can compensate for deviating pH values (e.g. due to citric acid). The faster the pH value rises again after rinsing with lemon juice (i.e. it becomes more basic), the better. The buffer capacity is poor if the pH value is still below 7.0 after 10 minutes (a pH of 8.0 should be aimed for).















.jpg)

.jpg)
