antibody
What are antibodies
Antibodies - also known as immunoglobulins or ab or Ig for short - are important components of the body's own defense system, which are formed by B cells or plasma cells, a subclass of lymphocytes.

It is a group of proteins formed by the human organism that serve to defend against foreign material. Normally, this exogenous material corresponds to pathogens such as bacteria, viruses or fungi. However, components of the red blood cells, the erythrocytes, can also be recognized and eliminated. A pathological immune response is found, for example, in an allergic reaction or in an autoimmune disease.
Depending on their function and place of production in the body, they can be divided into five classes: IgA, IgG, IgM, IgE, IgD, where Ig stands for immunoglobulin. This describes a group of proteins that also includes antibodies. The antibodies are part of the specific immune defense. This means that the antibodies are only responsible for a specific antigen. In contrast, the blood cells are part of the cellular immune defense, the unspecific immune response. More precisely, the antibodies are produced by B lymphocytes, a subgroup of leukocytes. The antibodies are able to recognize and bind antigens. The antigens are on the surface of the material to be eliminated. Each antibody has a specific binding site for a particular antigen. This means that every antibody can recognize and eliminate a certain antigen, the variety of antibodies is accordingly very large. In the case of immunodeficiencies, the formation of one or more antibodies can be reduced.
Read something Superantigens.
introduction
Antibodies are included Egg whites, which are composed of four different amino acid chains: two identical light and two identical heavy chains, but each antibody is different and individual and has a highly specific task in the immune system holds.
Each antibody formed can only recognize, bind (lock and key principle) and fight against very special structures, so that specific antibodies are formed for every foreign substance and every pathogen that infects the body and in the blood or are present in other body fluids.
The antibodies already acquire this specialization when they are formed by the B cells / plasma cells: the latter come into contact with an antigen (e.g. pathogens such as bacteria or viruses) or are caused by other immune cells (T cells) that had an antigen contact are activated so that they immediately begin to produce antibodies that have exactly the binding site that is necessary to capture the antigens from the blood.
When finished, they are released freely into the blood by the B cells, where they then search for "their" antigens in order to bind them and thus make other immune cells, such as the phagocytes, accessible for destruction.
The body's own antibodies of the immune system are divided into 5 subclasses, the immunoglobulins G, M., A., E., and D..
Artificially produced antibodies or antibodies obtained from animals can also be supplied to the body from outside, e.g. as part of a therapy for diseases with a disturbed or missing immune system, as a passive vaccine against various pathogens or for various types of cancer.
Structure of the antibodies
The structure of each antibody is usually the same and consists of four different amino acid chains (amino acids are the smallest building blocks of proteins), two of which are known as heavy chains and two as light chains. The two light and the two heavy chains are completely identical and are connected to one another by molecular bridges (disulfide bridges) and brought into the characteristic Y-shape of an antibody.
The light and heavy chains consist of constant amino acid segments, which are the same in all different antibody classes, and variable segments that differ from antibody to antibody (IgG therefore has a different variable segment than IgE).
The variable domains of the light and heavy chains together form the respective specific binding site for the antigens that match the antibodies (any structures or substances in the body).
In the area of the constant part, there is a second binding site (Fc part) for each individual antibody, which is not intended for an antigen, but rather a binding site with which they bind to certain cells of the immune system and activate their function can.
Role of antibodies
Antibodies are structures made up of proteins that are formed by the immune system. They serve the Recognition and binding of foreign cell structures.
They look like a "Y". With the two short, upper arms you can bind the foreign cells. They either use both or just one arm. If you only use one arm, you can bind to another antibody with the other arm. When this happens to multiple antibodies, they clump together and can be eaten by macrophages. The macrophages then break down these clusters, thereby destroying the foreign cells.
If you use both upper arms, you can use your lower arm directly to reach other cells of the Immune system, how T helper cells, tie. The T-helper cells then take up the antibodies, break them down and build the foreign cell components into their own membrane. In this way they act as information cells for other immune cells. Antibodies help roughly with this recognize foreign cells and allow other cells to destroy it. So they serve as a kind Link between the immune cells.
Antibodies in the blood
If a pathogen or another foreign substance (antigen) gets into the human body (e.g. via the skin or the mucous membranes), it is initially removed from the "superficial" ones Defense cells of the immune system (so-called. dendritic cells) recognized and bound to then move to the deeper ones Lymph nodes to hike. There the dendritic cells show the antigen to the so-called T lymphocytes, a class of white blood cells. These are thereby awakened to "helper cells" and in turn activate the B lymphocytes, which immediately start producing antibodies that are precisely tailored to the antigen to be rendered harmless. When these antibodies are fully formed, they are released into the circulating blood so that they can reach all parts of the body with the physiological bloodstream.
Another possibility of B-cell activation is direct contact A B cell swimming in the blood with the pathogen or the foreign substance, without prior activation by a T cell. The antibodies released into the blood (also Immunoglobulins called) can generally be divided into different classes (IgG, IgM, IgA, IgD and IgE) and can be determined by a blood sample and subsequent medical laboratory tests.
What are antigens?
Antigens are structures or substances on the surface of cells in the human body. They are mostly proteins, but can also be fats, carbohydrates or even completely different compositions.
Either these are the body's own structures, which are always present in the human body under normal circumstances, or foreign structures or substances that have entered the body but do not actually belong there.
These foreign antigens are usually recognized by the B or T lymphocytes of the immune system and are bound and rendered harmless by specific antibodies that have previously been formed by the B lymphocytes. Right from the start, the immune system learns to distinguish the body's own structures from foreign ones, so that only foreign antigens are fought under healthy circumstances. However, if the immune system falsely recognizes the body's own harmless structures as foreign antigens and also fights them, this pathological process is called an autoimmune reaction, from which autoimmune diseases can arise.
Read more on the topic: What is an autoimmune disease?
Function of antibodies
The main job of antibodies is to get into the body Pathogens or foreign substances or substances too detect, to tie and to destroy.
Those of the B lymphocytes (a certain subspecies of the white blood cells) produced protein molecules can be divided into different antibody classes, each of which has different tasks and properties and in some cases also has their main place of action in different parts of the body.
If the pathogen or the foreign molecule (antigen) in the body is recognized by the immune system, the B cells immediately start producing the appropriate antibody, which then docks with one connection point to the structure to be combated and with the other connection point to other defense cells of the body (e.g. macrophages = phagocytes).
These are then activated and absorb the antibody-antigen complexes, rendering the foreign substances or pathogens harmless.
Antibody screening test
The antibody search test (AKS for short) is a test in laboratory medicine in which the patient's blood serum is searched for certain antibodies that are against specific structures (antigens) on the membrane red blood cells (Erythrocytes) are directed. A distinction is made here regular and irregular antibodies against the red blood cells: the regular ones are the so-called Anti-A and Anti-B Antibodies, whereby the anti-A antibody is present in patients with blood group B, the anti-B antibody correspondingly in patients with blood group A. The irregular antibodies include, among others, of the Anti-D antibodieswhich is directed against the rhesus factor-D.
In order to find the regular and irregular antibodies in the patient's blood serum, the patient's serum is mixed with the corresponding antigens after the blood sample has been taken, so that if the antibodies are present, the blood clots: the test is then called positive rated. The antibody screening test is primarily used as a preparation for upcoming Blood transfusions carried out as well as within the Pregnancy check-ups. In everyday clinical practice, the term "antibody search test" is also used generally for the determination of antibodies in the context of e.g. Infectious or autoimmune diseases are used, but should not be confused with the actual meaning as described above.
Antibody treatment
As described above, antibodies actually serve to protect against disease and are therefore part of the immune system. However, our immune system cannot fight some diseases, such as cancer, on its own because it is not fast and effective enough to do this.
For some of these diseases one got through many years of research Antibodies foundthat can be produced biotechnologically and then given as medication to patients, for example cancer patients. That brings huge advantages. While chemotherapy or radiation therapy attacks the entire body and destroys all cells, including healthy cells, are effective Antibodies only very specifically against the cancer cells.
This specificity is due to the nature of the antibodies. Antibodies are proteins that are normally produced by cells of the immune system. Before these cells of the immune system, the plasma cells, can do this, however, they must have come into contact with the foreign cells. To do this, they take in the foreign cells, break them down and recognize superficial structures that "identify" the cells, like an identity card. Antibodies are then formed against these superficial structures, also called surface markers.
This principle has been used in research. One has Cancer cells searched for such surface markers, the only on the cancer cells can be found, but not on the body's own cells. Against these markers were then Antibodies formedthat can be given to patients in the form of antibody treatment. The antibodies then bind to the cancer cells in the body and thus help the body's own immune system to recognize and kill the malignant cells.
This is how the antibody works Rituximab with certain types of leukemia and the Non-Hodgkin lymphoma and the antibody Trastuzumab against Breast cancer cells and some Stomach cancer cells. In addition to these relatively “disease-specific antibodies”, there are also those that, for example, inhibit the growth of new blood vessels and thus prevent the cancer from being supplied with nutrients from the blood. That would be such an antibody Bevacizumab. It can be used in many different types of cancer.
Immunoglobulins IgG, IgM, IgA, IgE
The antibodies formed by the B lymphocytes, also called immunoglobulins, can generally be viewed in 5 subclasses to be grouped: Immunoglobulin M (IgM), Immunoglobulin G (IgG), Immunoglobulin A (IgA), Immunoglobulin E. (IgE) and Immunoglobulin D (IgD).
The different Antibody subclasses have different tasks in the immune system and also differ in the main location (free, dissolved in the blood or in other body fluids as well as on the membrane of immune cells).
Type a
The IgA is mainly found in body fluids and on mucous membranes. The mucous membrane of the mouth and the saliva, the mucous membrane of the respiratory tract, the mucous membrane of the gastrointestinal tract and gastric juice and the vaginal mucous membrane are important here. The IgA prevents pathogens from entering the organism through non-intact mucous membranes. This function is particularly important in the non-sterile areas of the body as well as the body orifices that are in constant contact with the environment, e.g. Mouth and nose. In addition, IgA is involved in eliminating pathogens that we ingest daily with food, liquids or breath. IgA is also found in breast milk. Through breastfeeding, antibodies from the mother are transferred to the child and thus ensure the child's immunity to pathogens without the infant coming into contact with the pathogen. This mechanism is known as nest protection.
Type D
Immunoglobulins from Type D also almost never occur freely in the blood plasma. Rather, they come bound on the membrane of B lymphocytes where they form a kind of receptor for certain antigens, through which the B cells are stimulated to further produce antibodies.
Type E
IgE is of particular importance in the development of allergies. IgE is formed by B lymphocytes when they first come into contact with an allergen, such as pollen in hay fever. If the IgE is formed, renewed contact with the inhaled pollen leads to an allergic reaction. The IgE stimulates histamine-containing mast cells so that the histamine is released.
Depending on the strength of the reaction and depending on the location of the allergen, the histamine will cause symptoms. The symptoms of hay fever may include burning, itchy eyes, a runny, itchy nose, or shortness of breath. In the worst case, the allergic reaction leads to anaphylactic shock, which is characterized by shortness of breath, swelling of the airways, a drop in blood pressure as a sign of shock and unconsciousness. This is a medical emergency and requires immediate medical attention. The allergic symptoms can be alleviated with histamine blockers. These block the receptors for histamine so that the histamine does not have any effect after it is released. One of the main side effects of histamine blockers is fatigue.
Another task of IgE antibodies is to eliminate parasites.
Type G
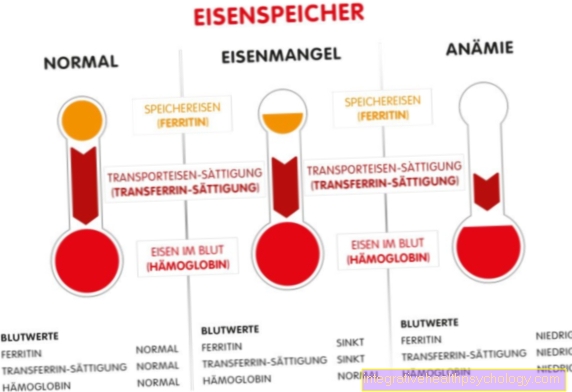
In terms of quantity, IgG takes up the largest proportion of the antibodies. The IgG is formed in the course of the infection and is therefore part of the late immune response. If the IgG is present in the blood, it can be concluded that the infection has passed or has just subsided; full immunity is guaranteed by the IgG. Because the immune system “remembers” the antibodies it has produced, in the event of a reinfection with the same pathogen, the antibodies can be quickly reproduced and the infection with symptoms of the disease does not break out.
The special thing about the IgG is that this antibody crosses the placenta. The unborn child can thus receive IgG antibodies from the mother and is immune to pathogens without having come into contact with them. This is called nest protection. However, rhesus antibodies are also IgG antibodies and are therefore plant day-long. If a rhesus-negative mother has antibodies against the rhesus factor from the rhesus-positive erythrocytes of the child, these antibodies can be transferred to the child in the subsequent pregnancy and destroy the child's erythrocytes. This leads to the breakdown of red blood cells, also known as hemolysis, which leads to anemia (anemia) in children. The clinical picture in infants is called Morbus hemolyticus neonatorum. In rhesus-negative mothers with a rhesus-positive child father, passive immunization with anti-D antibodies (rhesus prophylaxis) can be carried out during pregnancy.
Type M
The IgM (immunoglobulin M) is structurally the largest antibody. It is formed when new infections occur and is involved in quickly eliminating pathogens and preventing them from spreading. IgM antibodies in the blood indicate an ongoing, fresh infection.
The IgM antibody also has a binding site for other systems of the immune system. A part of the complement system, which consists of around twenty proteins and also serves to defend against infection, can bind to the antibody-antigen complex. This is how the complement system is activated. The antibodies against a foreign blood group, which are formed, for example, during a blood transfusion with the wrong blood group, are also IgM antibodies. These lead to a reaction to the foreign blood and cause the blood to thicken (coagulation). This can have serious consequences for the person concerned and even be fatal within a very short time. Therefore, before a blood transfusion, careful attention should be paid to the matching of the blood groups of the donor and recipient. This is guaranteed by the so-called “bedside test”, in which the donor's blood is mixed with that of the recipient immediately before the transfusion and is observed. If there is no reaction, the blood can be transfused.
Auto antibodies
Auto-antibodies are antibodies that the body produces in order to recognize and bind with the body's own cells in tissues, hormones or other antibodies. By binding the auto-antibodies to these structures, the immune system is activated and fights these structures.
Auto-antibodies are formed in the course of autoimmune diseases. Auto-antibodies do not help our immune system to remove foreign bacteria or viruses from our body, as normal antibodies do, but attack our own body. Whenever the immune system forms auto-antibodies against its own body, it is extremely pathological and leads to the destruction of actually healthy tissue.
This destruction in turn results in the loss of tasks that the tissue should actually take over. The immune system makes the body sick instead of keeping it healthy and functional. Many different auto-antibodies are known which, depending on which structure they attack, trigger different diseases. Examples of such diseases include type I diabetes mellitus, which can be caused by four different auto-antibodies. But lupus erythematosus or rheumatoid arthritis are also caused by auto-antibodies.
Hashimoto's disease
Because Hashimoto's thyroiditis to the Autoimmune diseases counts, antibodies specific for this disease are usually present in the blood serum of the affected patient, which can be determined by means of a blood sample and a laboratory test and the amount that can be measured. On the one hand, this serves to make a diagnosis of Hashimoto's disease if there is initially only a suspicion. On the other hand, this is also used to monitor the progress and to observe an already fully diagnosed, existing Hashimoto thyroid inflammation.
The characteristic antibodies in this disease are the so-called Thyroglobulin antibodies (Tg-Ak) and the Thyroid peroxidase antibodies (TPO-Ak). The Tg antibodies are directed against that Thyroglobulin of the thyroid gland, a protein that is made by thyroid cells and with the help of which the Thyroid hormones stored in the blood before being released.
The TPO antibodies however, are directed against the thyroid enzyme thyroid peroxidase, which is involved in the formation of thyroid hormones. In about 10-20% of Hashimoto's patients, these antibodies are not found in the blood, although Hashimoto's disease is present.
Unlike the Basedow's thyroid disease it is not assumed that these auto-antibodies against the thyroid tissue in Hashimoto's disease are responsible for the damage or destruction of the thyroid, as these are often only increased in phases and the level of the antibody levels does not correlate with the disease intensity.








.jpg)