Trypsinogen
Definition - What is Trypsinogen?
Trypsinogen is the inactive precursor, a so-called Proenzyme, an enzyme made in the pancreas, called the pancreas. Together with the remaining pancreatic secretion, the so-called pancreas, the proenzyme trypsinogen is released via the pancreatic ducts into the duodenum, part of the small intestine. This is where activation to the enzyme trypsin takes place.
This enzyme is called "Hydrolase"categorized, that is, it is able to split connections between individual amino acids. This process takes place in the Small intestine instead, which breaks down the proteins that are ingested through food into smaller fragments of amino acids, which enables them to be absorbed into the body.

How is activation to trypsin done?
The activation of trypsinogen to trypsin can take place in two different ways. In both ways, activation does not take place in the area of the pancreas or its ducts, but only in the area of the duodenum, a part of the small intestine.
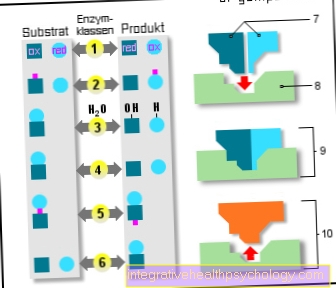
- Another enzyme is required for one possibility of activation to trypsin. This enzyme is produced in the brush border, i.e. the superficial cells, of the duodenum. It's called Enteropeptidase or Enterokinase. The enzyme is among the Hydrolases categorized. This means that they can reversibly cleave the compounds of individual amino acids, which give the proenzyme trypsinogen its structure, by consuming water molecules. When trypsinogen is activated to trypsin, a chain of six amino acids, a so-called hexapeptide, is split off from the proenzyme trypsinogen while consuming water. This creates a shortened amino acid chain compared to before. The process is called limited proteolysis. However, the enzyme is now in its active form and can split further amino acid chains in order to be able to break down and digest proteins.
- The second variant of activating trypsinogen to trypsin is represented by the already active enzyme trypsin. Trypsin can not only split foreign proteins into smaller amino acid chains, but can also shorten the body's own proenzymes such as trypsinogen by several amino acids. Trypsin especially likes to split after the sixth amino acid of trypsinogen. This means that a hexapeptide is split off, which converts the trypsinogen into its active form, trypsin. In addition to trypsinogen, active trypsin can convert three other enzymes that are important for digestion into their active form. Two factors that are not initially obvious are also important for activation. On the one hand, the effect of trypsin is particularly good at a slightly basic pH value of 7 to 8, which means that trypsinogen is increasingly activated. On the other hand, trypsinogen is released in the pancreas with a trypsin inhibitor. This prevents premature activation within the pancreas and is only broken down in the duodenum.
Where is trypsinogen made?
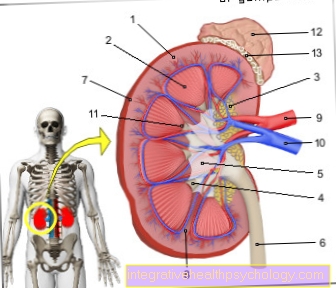
The proenzyme trypsinogen is roughly formulated in the pancreas. This lies across the upper abdomen to the left of the stomach. The pancreas can still be divided into two parts:
- Of the endocrine part produces hormones like insulin for the regulation of the sugar balance, which work within the body.
- Of the exocrine partwhich makes up the bulk of the pancreas, produces the pancreas, which is the proenzyme Trypsinogen and plays an important role in digestion.
You might also be interested in: Functions of the pancreas
What are the normal values?
Since trypsinogen is normally passed on directly into the small intestine via the ducts of the pancreas, there is usually no trypsinogen in the blood, which means that the normal values approach zero.
If it is the case that trypsinogen is detected in the blood, the finding definitely speaks for a pathological process. Here, for example, comes an acute one Pancreatitis and a Cystic fibrosis in question. Trypsin is tested as part of the newborn screening.
What does trypsinogen have to do with cystic fibrosis?
In cystic fibrosis, also called cystic fibrosis, a mutation in the genome results in a change in the composition of the secretion from glands that release their secretion to the body surface such as the intestine. The secretion becomes significantly more viscous, which means that it can be released more slowly.
This is particularly critical in the case of the pancreas. Due to the longer residence time in the passages of the pancreas, the secretion has an increased effect within the organ. Since trypsinogen is also increasingly activated to trypsin, digestion of the own body occurs, which can result in acute pancreatitis.
Further information on the topic can be found here: Cystic fibrosis
What is trypsin?
Trypsin is an enzyme that arises from an inactive precursor, the proenzyme trypsinogen, and plays an important role in the digestion of proteins. The proenzyme trypsinogen comes from the exocrine part of the pancreas. This proenzyme is activated in two different ways. On the one hand, with the aid of the enzyme enteropeptidase, an amino acid chain made up of six amino acids is split off. On the other hand, trypsin can activate itself. Here, too, an amino acid chain made up of six amino acids is split off. Active trypsin can also do the three proenzymes Procarboxypeptidases, Proaminopeptidases and Chymotrypsinogen convert to their three active enzymes by splitting off an amino acid chain. These three enzymes are also involved in the digestion of proteins.
Trypsin is classified as an enzyme under the category of Hydrolases categorized. This means that they can reversibly split connections between amino acids by consuming water. The ability to split amino acid chains reaches a maximum in the slightly basic pancreas with pH values between 7 and 8. This property is essential for the digestive process.
After the enzymes in the saliva of the mouth, trypsin represents the second step in the cleavage of proteins. The enzyme does not cleave the amino acid chain of the proteins from the outside, but rather divides the entire chain into several small fragments, which are then shortened by other enzymes so that they are can be absorbed into the body through the intestinal mucosa.
More information can be found here: Trypsin
What happens with a trypsin deficiency?
With a lack of trypsin, the digestion of proteins is disturbed. In the following, fewer amino acids are absorbed into the body. Since some amino acids are essential for the human body, as they cannot be produced either by modification of existing amino acids or by their own synthesis, deficiency symptoms occur after a while, which if left untreated can have serious consequences.
In addition, it can happen that the body's amino acid stores, such as the proteins in the muscles, are used, which leads to weight loss and decreased resilience.
Alpha-1 antitrypsin deficiency
Alpha-1 antitrypsin deficiency is often caused by a genetic defect. Alpha-1-antitrypsin is an enzyme that inhibits other enzymes from working. The enzymes that are inhibited in the process normally have the task of breaking down proteins, making them lose their function. Thus, Alpha-1-Antitrypsin can also be used as Proteinase inhibitor are designated.
The enzymes that are inhibited by Alpha-1-Antitrypsin occur mainly in inflammatory processes and are mainly Chymotrypsin, trypsin, plasmin, elastase and Thrombin.
The inhibition of elastase is of particular importance here. As a rule, elastase breaks down elastin, which is mainly found in the lungs. Elastin is a structural protein that is largely responsible for the elasticity of the lungs. If there is a lack of elastase inhibitors such as alpha-1-antitrypsin, this can lead to increased activity of elastase in the lungs. Here, like everywhere else in the body, elastase breaks down proteins, but this affects the body's own tissue in the lungs. This leads to massive damage to the lung tissue, which inevitably restricts lung function. The symptoms that develop from it include Cough, shortness of breath and a narrowing of the airways. In addition, the liver, which is mainly responsible for the production of elastase, can show elevated liver values and be further damaged by biliary congestion.
More information can be found here: Alpha-1 antitrypsin deficiency
Recommendations from our editorial team
- Trypsin
- Alpha-1 antitrypsin
- Alpha-1 antitrypsin deficiency
- Chymotrypsin - What Is It Important For?
- Role of enzymes in the human body