The tyrosine kinase
What is a tyrosine kinase?
Tyrosine kinase is a specific group of enzymes that are functionally assigned to protein kinases from a biochemical point of view. Protein kinases transfer reversibly (possibility of reverse reaction) phosphate groups to the OH group (hydroxyl group) of the amino acid tyrosine. The phosphate group is transferred to the hydroxyl group of the tyrosine of another protein.
Through this reversible phosphorylation described, tyrosine kinases can decisively influence the activity of proteins and therefore play an important role in signal transduction pathways. Especially therapeutic, such as In oncology, the function of tyrosine kinases is used as a target for drugs.

The task and function
Tyrosine kinases must first be subdivided into membrane-bound and non-membrane-bound tyrosine kinases in order to understand how they function.
Membrane-bound tyrosine kinases can have their own protein kinase activity, the kinase function being activated as part of the receptor complex on the cell membrane. Otherwise, membrane-bound tyrosine kinases can be functionally linked to the receptor complex, but cannot be directly localized in it. Here, the tyrosine kinase and the receptor create a bond via which a certain signal is passed on to the kinase via the receptor.
In the case of a non-membrane bound tyrosine kinase, this is either in the cytoplasm or in the nucleus of a cell. Different examples of tyrosine kinases can be named depending on the structural design with an associated function. Examples of membrane-bound tyrosine kinases are the insulin receptor, the EGF receptor, the NGF receptor or the PDGF receptor. This shows that the signal cascades with the help of tyrosine kinases are vital processes in the human body.
The insulin release from the pancreas in connection with meals is regulated via the insulin receptor. The EGF receptor has specific binding sites for several ligands, among which EGF or TNF-alpha are worth mentioning. As a protein ligand, the EGF (epidermal growth factor) takes on an outstanding role as a growth factor (cell proliferation and differentiation). The TNF-alpha, on the other hand, is one of the strongest inflammation-promoting markers in the human body and plays an important diagnostic role in the diagnosis of inflammation.
PDGF, in turn, is a growth factor released by thrombocytes (blood platelets), which induces wound closure and, according to current research, also contributes to the development of pulmonary hypertension.
Examples of non-membrane-bound tyrosine kinases are the ABL1 and the Janus kinases.
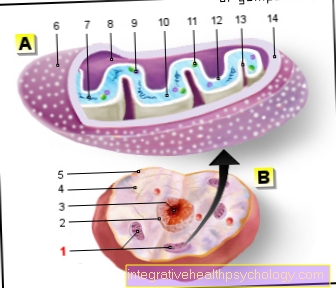
In principle, a signal cascade with certain information always proceeds in the same stereotypical manner in the case of a tyrosine kinase. First, a suitable ligand must bind to a receptor, which is usually located on the surface of cells. This link is usually established via a congruent protein structure of ligand and receptor (lock and key principle) or via binding to certain chemical groups of the receptor (phosphate, sulfate groups, etc.). The linkage changes the protein structure of the receptor. In the case of tyrosine kinases in particular, the receptor forms homodimers (two identical protein subunits) or heterodimers (two different protein subunits). This so-called dimerization can lead to an activation of tyrosine kinases, which, as already mentioned above, are located directly in the receptor or on the cytoplasmic side (facing the cell interior) of the receptor.
Activation links the hydroxyl groups of tyrosine residues of the receptor with phosphate groups (phosphorylation). This phosphorylation creates recognition sites for intracellularly localized proteins that can subsequently bind to them. They do this via specific sequences (SH2 domains). After binding to the phosphate groups, highly complex signal cascades are triggered in the cell nucleus, which in turn leads to phosphorylation.
It should be noted that the activity of proteins can be influenced in both directions via phosphorylation by tyrosine kinases. On the one hand these can be activated, on the other hand they can also be deactivated. It can be seen that an imbalance of the tyrosine kinase activity can lead to an overstimulation of growth factor-associated processes, which ultimately allows body cells to multiply and dedifferentiate (loss of cellular genetic material). These are the classic processes of tumor development.
Defective regulatory mechanisms of tyrosine kinases also play a decisive role in the development of diabetes mellitus (insulin receptor), arteriosclerosis, pulmonary hypertension, certain forms of leukemia (especially CML) or non-small cell lung cancer (NSCLC).
Find out all about the topic here: Tumor diseases.
What is the tyrosine kinase receptor?
The tyrosine kinase receptor is a membrane-based receptor, that is, a receptor anchored in the cell membrane. Structurally, this is a receptor with a transmembrane complex. This means that the receptor pulls through the entire cell membrane and also has an extra- and intracellular side.
On the extracellular side, the alpha subunit, the specific ligand binds to the receptor, while the catalytic center of the receptor is located on the intracellular side, the ß subunit. The catalytic center represents the active area of the enzyme, where specific reactions take place.
As already mentioned above, the structure of the receptor is usually composed of two protein subunits (dimers).
With the insulin receptor e.g. the two alpha subunits bind the ligand insulin. After ligand binding, phosphate groups (so-called phosphorylation) are bound to specific tyrosine residues (hydroxyl groups). This generated the tyrosine kinase activity of the receptor.In the following, further substrate proteins (e.g. enzymes or cytokines) inside the cell can be activated or inactivated via renewed phosphorylation, thereby influencing cell proliferation and differentiation.
What is a tyrosine kinase inhibitor?
So-called tyrosine kinase inhibitors (also: tyrosine kinase inhibitors) are relatively new drugs that can be used to specifically treat defective tyrosine kinase activity. They are assigned to the chemotherapy drugs and have their origin in the late 1990s and early 2000s. They can be classified into different generations and are used in the treatment of malignant diseases.
Functionally, specific processes can be prevented by unbalanced tyrosine kinase activities. In principle, four different mechanisms of action are possible here. In addition to competing with ATP, binding to the phosphorylating unit of the receptor, to the substrate or allosterically outside the active center is also possible. The action of the tyrosine kinase inhibitors is triggered by a binding to the EGF receptor and the subsequent inhibition of the enzymatic activity of the tyrosine kinases.
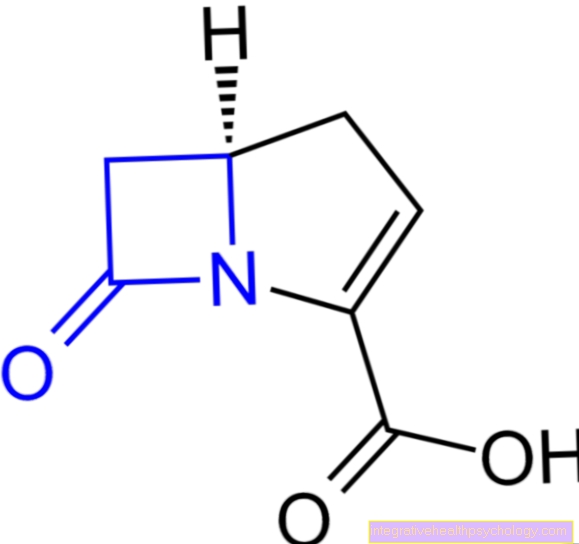
In medical history, the discovery of the active ingredient imatinib as a tyrosine kinase inhibitor achieved an outstanding position. It is used specifically in chronic myeloid leukemia (CML), where it suppresses the tyrosine kinase activity that is pathologically produced by a chromosome fusion (Philadelphia chromosome by fusion of chromosome 9 and 22).
Several more tyrosine kinase inhibitors have been developed in recent years. The currently existing 2nd generation contains around ten tyrosine kinase inhibitors.
Read more about the topic here:
- Targeted chemotherapy with tyrosine kinase inhibitors
- The chronic myeloid leukemia.
For which indications are they used?
Tyrosine kinase inhibitors are used for various malignant diseases. Imatinib is used in particular in chronic myeloid leukemia. Other possible uses are non-small cell lung cancer (NSCLC), breast cancer and colon cancer.
Due to the very selective attack mechanism of the tyrosine kinase inhibitors, they are usually better tolerated than conventional chemotherapeutic agents. Nevertheless, side effects are to be expected here as well.
Find out more about: Lung cancer.